Draw the structure of the predominant form of
(e) a mixture of alanine, lysine, and aspartic acid at (iii) pH 2.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: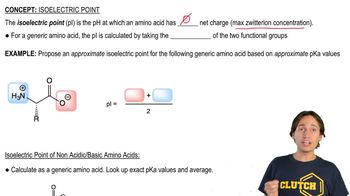
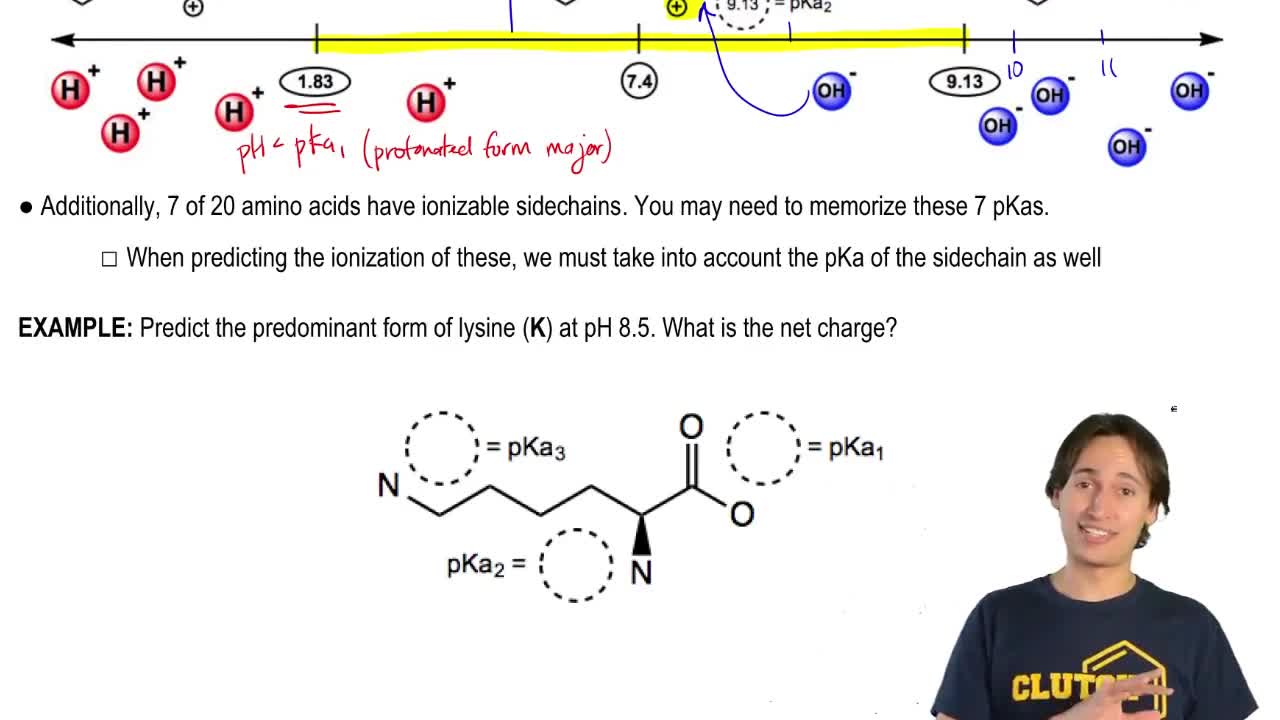

 4:41m
4:41mMaster Why Amino Acids Exist as Zwitterions with a bite sized video explanation from Johnny
Start learning