Which of molecules A–D would you expect to give a positive permanganate test? That is, which would result in a purple KMnO₄ solution turning brown?
(d)
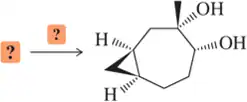
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:50m
3:50mMaster General properties of syn vicinal dihydroxylation. with a bite sized video explanation from Johnny
Start learning