Textbook Question
Suppose a molecule is formed by the overlap of 16 atomic orbitals.
(a) How many molecular orbitals will be present?
(b) How many will be bonding?
(c) How many will be antibonding?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: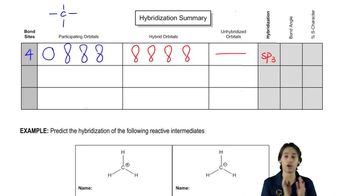

 6:00m
6:00mMaster Single bonds, double bonds, and triple bonds. with a bite sized video explanation from Johnny
Start learning