a. Is (R)-lactic acid dextrorotatory or levorotatory?
b. Is (R)-sodium lactate dextrorotatory or levorotatory?
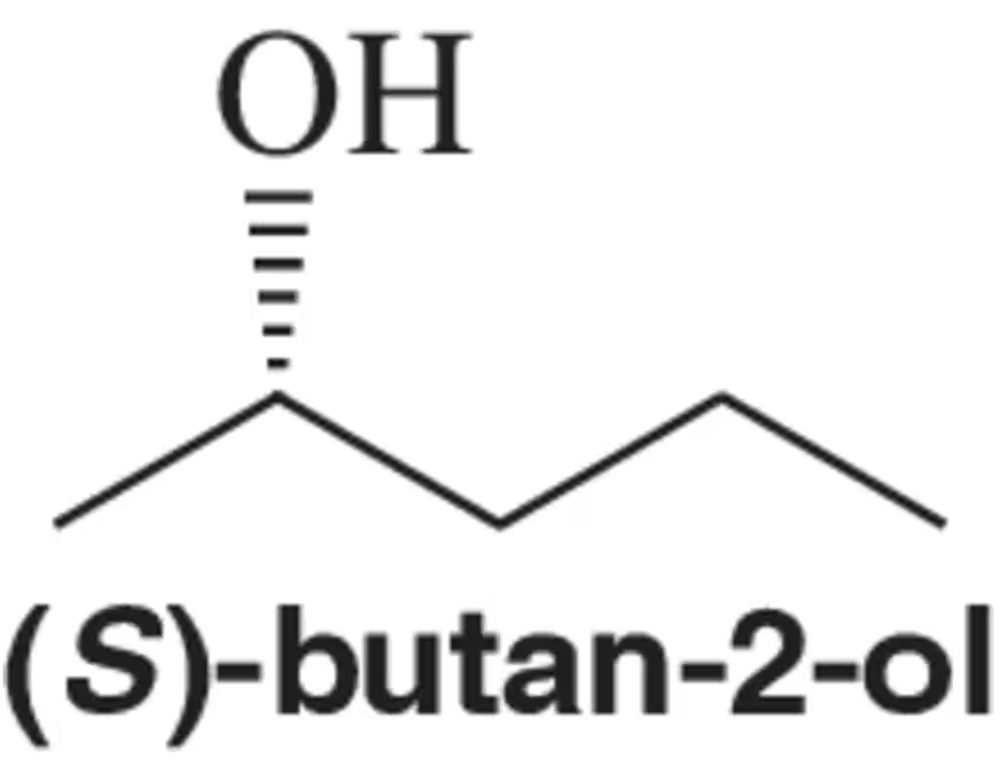
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: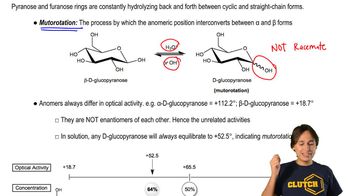


 5:43m
5:43mMaster Specific rotation vs. observed rotation. with a bite sized video explanation from Johnny
Start learning