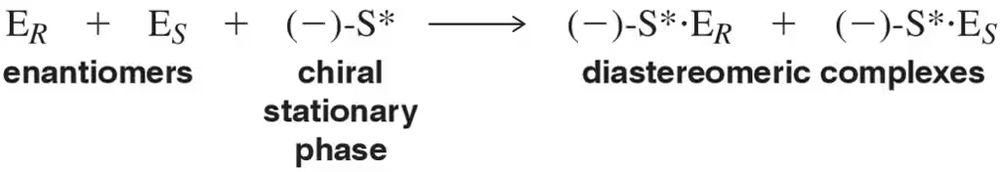
Questions (a)–(d) all refer to the following reaction, which has been engineered to produce one enantiomer to the exclusion of the other.
(c) Suppose the difference in activation energy is 1.6 kcal/mol. At what temperature would you produce C in 99% ee?