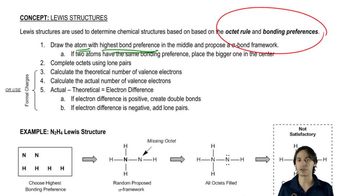
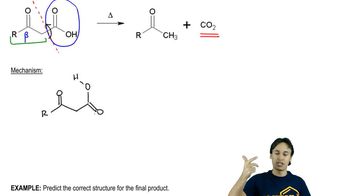
Textbook Question
When ethyl 4-hydroxybutyrate is heated in the presence of a trace of a basic catalyst (sodium acetate), one of the products is a lactone. Propose a mechanism for formation of this lactone.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: