Multiple Choice
Which of the following statements is true in regard to the peptide strand shown?
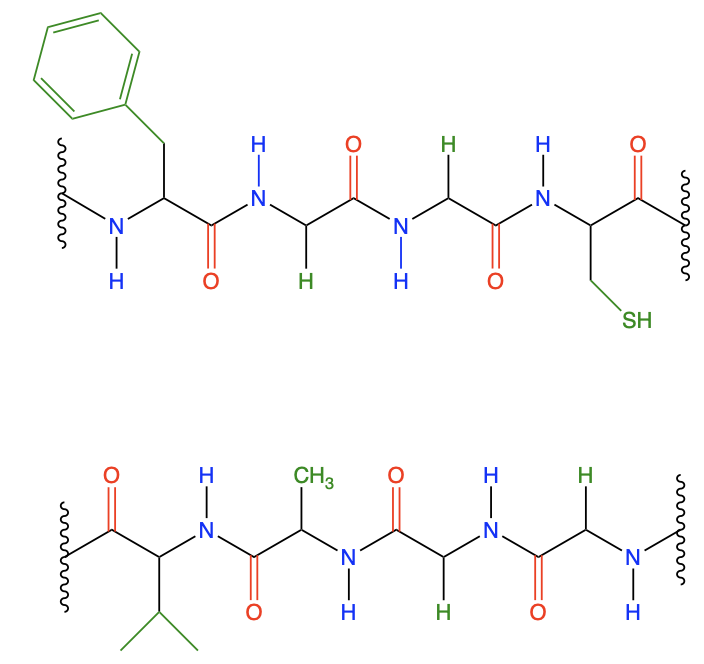
3
5
4
6
1
 Verified step by step guidance
Verified step by step guidance
 1:5m
1:5mMaster Secondary Protein Structure Concept 1 with a bite sized video explanation from Johnny
Start learning
