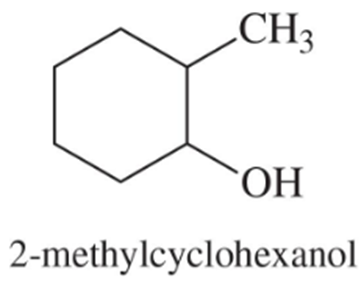
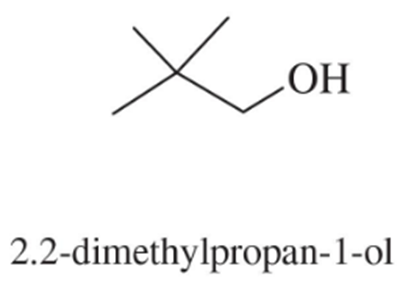
A student was studying terpene synthesis, and she wanted to make the compound shown here. First she converted 3-bromo-6-methylcyclohexene to alcohol A. She heated alcohol A with sulfuric acid and purified one of the components (compound B) from the resulting mixture. Compound B has the correct molecular formula for the desired product.
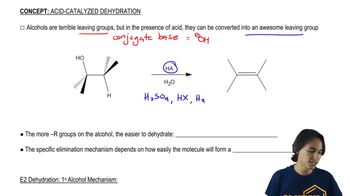
(c) Propose a mechanism for the dehydration of alcohol A to compound B.