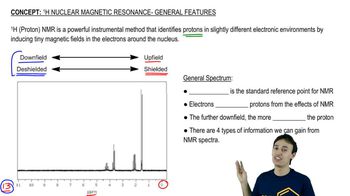
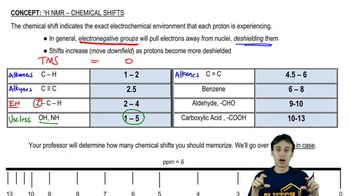
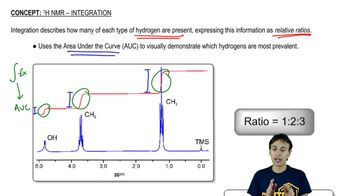
Propose structures that are consistent with the following spectra. (Integral ratios are given from left to right across the spectrum.)
b. The 1H NMR spectrum of a compound with molecular formula C6H10O2 has two singlets with integral ratios of 2 : 3.