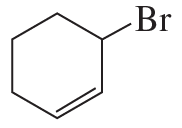
Show how you would synthesize each compound, starting with alkenes or cycloalkenes that contain no more than six carbon atoms. You may use any additional reagents you need.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: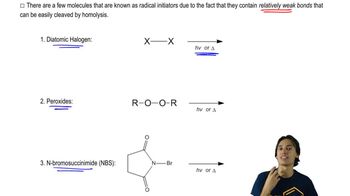

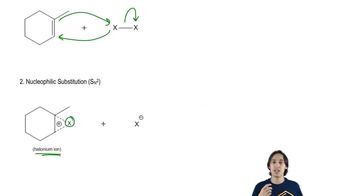

 4:39m
4:39mMaster Heterolytic vs. Homolytic Bond Cleavage . with a bite sized video explanation from Johnny
Start learning