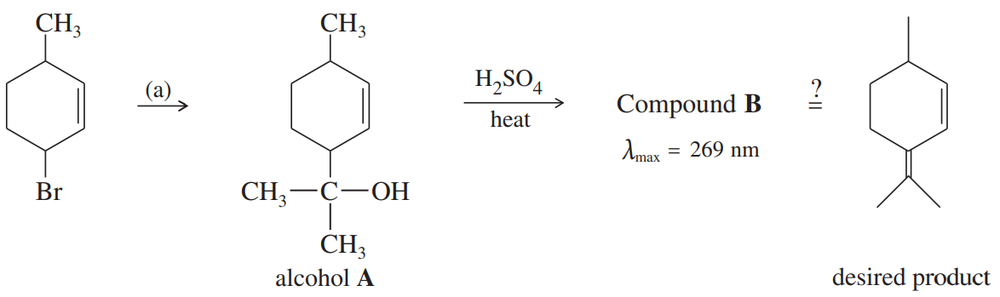
Predict the products of the following reactions.
(g) 1-(bromomethyl)-2-methylcyclopentene, heated in methanol
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 7:50m
7:50mMaster Allylic Halogentation - General Mechanism with a bite sized video explanation from Johnny
Start learning