a. How many linear dienes have molecular formula C6H10? (Disregard cis–trans isomers.)
b. How many of the linear dienes in part a are conjugated dienes?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: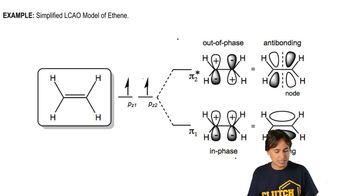

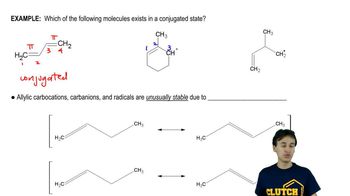

 5:29m
5:29mMaster Definition of Conjugation with a bite sized video explanation from Johnny
Start learning