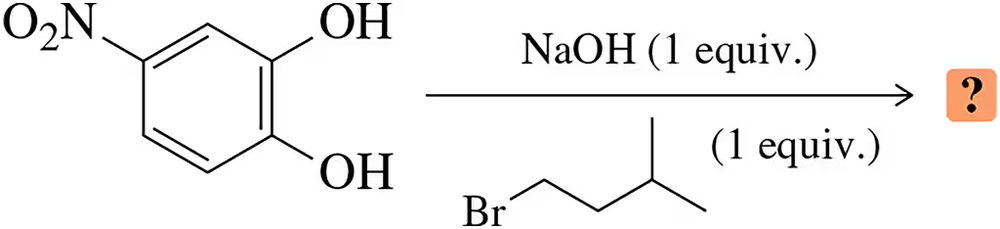
Show how the following ethers might be synthesized using (1) alkoxymercuration– demercuration and (2) the Williamson synthesis. (When one of these methods cannot be used for the given ether, point out why it will not work.)
a. 2-methoxybutane
b. ethyl cyclohexyl ether
c. 1-methoxy-2-methylcyclopentane