Given the reactants shown, what type of elimination would you expect to occur?
(a)
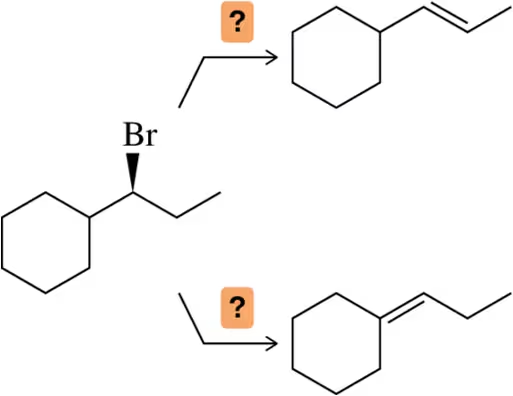
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: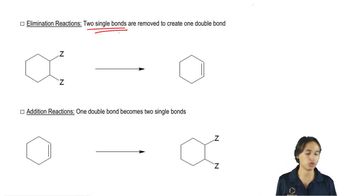


 2:27m
2:27mMaster Overview of the flowchart. with a bite sized video explanation from Johnny
Start learning