Predict the major and minor elimination products of the following proposed reactions (ignoring any possible substitutions for now). In each case, explain whether you expect the mechanism of the elimination to be E1 or E2.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
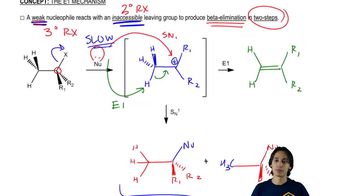

 2:27m
2:27mMaster Overview of the flowchart. with a bite sized video explanation from Johnny
Start learning