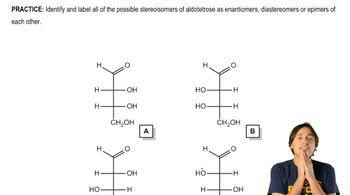
Textbook Question
Use Figure 23-3 (the D family of aldoses) to name the following aldoses.
(a) the C2 epimer of D-arabinose
(b) the C3 epimer of D-mannose
(c) the C3 epimer of D-threose
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: