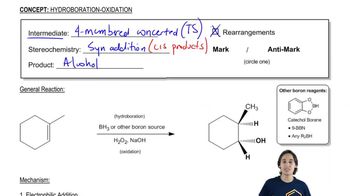
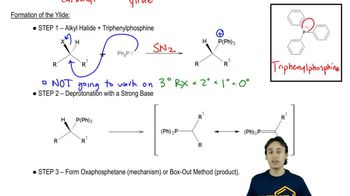
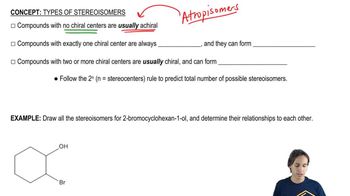
Disiamylborane adds only once to alkynes by virtue of its two bulky secondary isoamyl groups. Disiamylborane is prepared by the reaction of BH3·THF with an alkene.
a. Draw the structural formulas of the reagents and the products in the preparation of disiamylborane.
b. Explain why the reaction in part (a) goes only as far as the dialkylborane. Why is Sia3B not formed?