Identify the following molecules as chiral or achiral. If chiral, draw the nonsuperimposable mirror image and verify its nonsuperimposability.
(f)
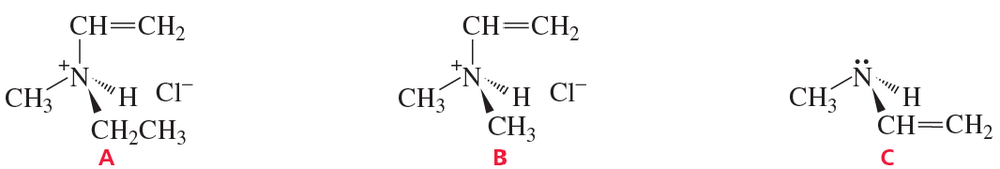
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 2:15m
2:15mMaster What is a stereocenter? with a bite sized video explanation from Johnny
Start learning