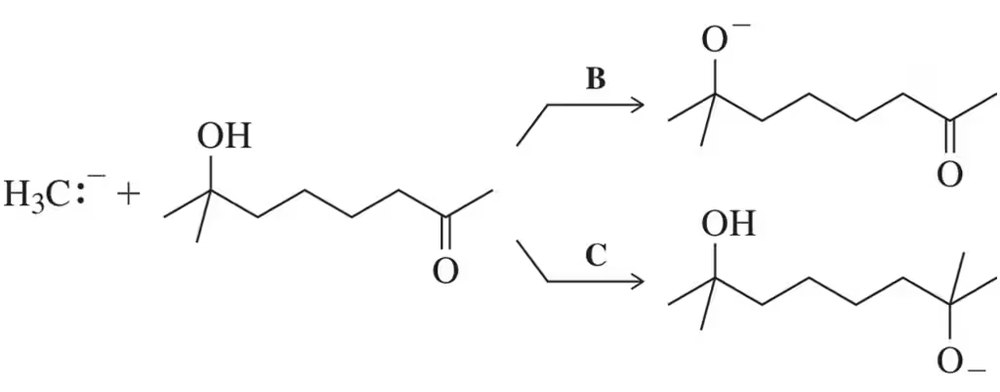
In a reaction in which reactant A is in equilibrium with product B at 25 °C, what relative amounts of A and B are present at equilibrium if ∆G° at 25 °C is
c. -2.72 kcal/mol?
d. -0.65 kcal/mol?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 5:02m
5:02mMaster Breaking down the different terms of the Gibbs Free Energy equation. with a bite sized video explanation from Johnny
Start learning