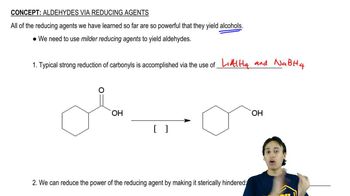
Show how you would accomplish the following synthetic transformations. You may use any necessary reagents.
(a) N-ethylbenzamide → benzylethylamine
(b) ethyl benzoate → N-ethylbenzamide
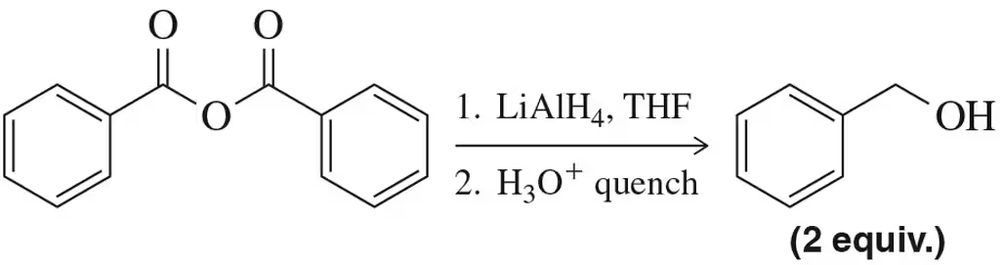
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: