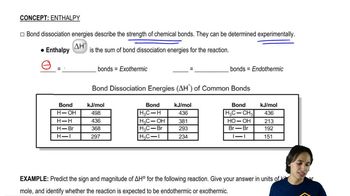
Based on the stability of the radicals produced, predict which bond in each pair would have the higher bond-dissociation energy.
(c)

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:43m
3:43mMaster The radical stability trend. with a bite sized video explanation from Johnny
Start learning