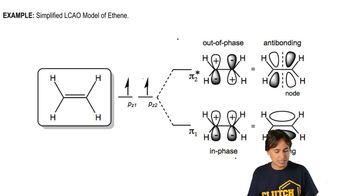
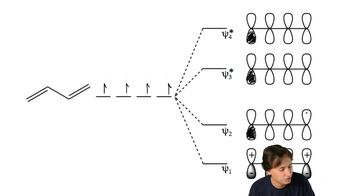
a. Use the polygon rule to draw an energy diagram (as in Figures 16-5 and 16-7) for the MOs of a planar cyclooctatetraenyl system.
b. Fill in the eight pi electrons for cyclooctatetraene. Is this electronic configuration aromatic or antiaromatic? Could the cyclooctatetraene system be aromatic if it gained or lost electrons?