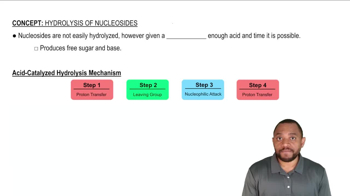
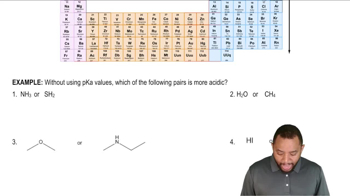
Methyl p-nitrobenzoate has been found to undergo saponification faster than methyl benzoate.
(a) Consider the mechanism of saponification, and explain the reasons for this rate enhancement.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: