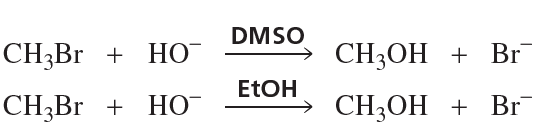
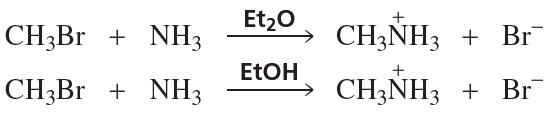
A solution of pure (S)-2-iodobutane ([α] = +15.90°) in acetone is allowed to react with radioactive iodide, 131I–, until 1.0% of the iodobutane contains radioactive iodine. The specific rotation of this recovered iodobutane is found to be +15.58°.
b. What does this result suggest about the mechanism of the reaction of 2-iodobutane with iodide ion?