What alcohols are formed from the reaction of ethylene oxide with the following organocuprates followed by the addition of acid?
b. (CH3CH=CH)2CuLi
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: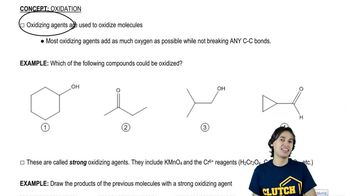



 0:24m
0:24mMaster Intro to Predict the Product with a bite sized video explanation from Johnny
Start learning