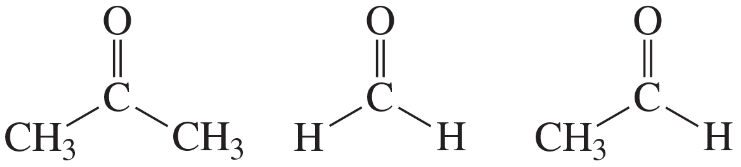
For each of the following pairs of compounds, name one absorption band that can be used to distinguish between them.
e.
f.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 16:47m
16:47mMaster Common IR Frequencies with a bite sized video explanation from Johnny
Start learning