a. What product is obtained from the reaction of HCl with 1-butene? With 2-butene?
b. Which of the two reactions has the greater free energy of activation?
c. Which compound reacts more rapidly with HCl: (Z)-2-butene or (E)-2-butene?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: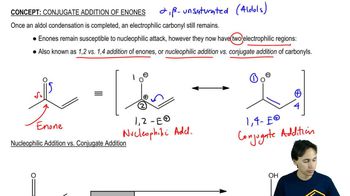



 4:07m
4:07mMaster General properties of hydrohalogenation. with a bite sized video explanation from Johnny
Start learning