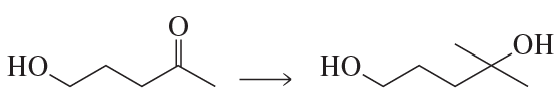
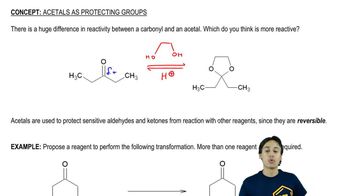
A chemist failed to generate the diol using the reaction shown here.
(a) Suggest a reason why this reaction did not work as written.
(b) How could the reaction conditions be modified to allow formation of the diol? [It may require more than one step.]