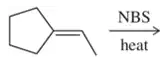
When N-bromosuccinimide is added to hex-1-ene in CCl4 and a sunlamp is shone on the mixture, three products result.
(a) Give the structures of these three products.
(b) Propose a mechanism that accounts for the formation of these three products
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
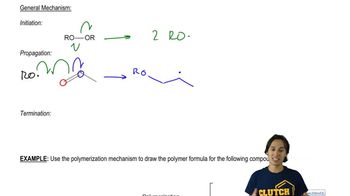


 6:15m
6:15mMaster The general mechanism of Allylic Halogenation. with a bite sized video explanation from Johnny
Start learning