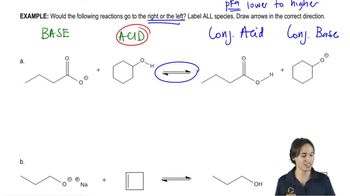
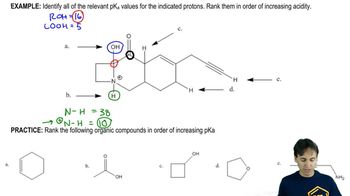
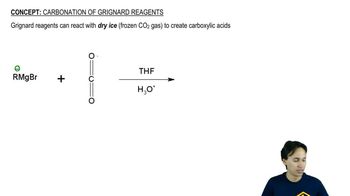
Complete the following acid–base reactions. In each case, indicate whether the equilibrium favors the reactants or the products, and explain your reasoning.
(e) (CH3)3C–O– + CH3CH2OH ⇌
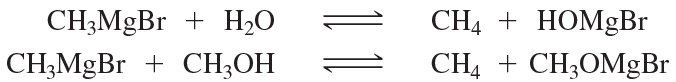
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: