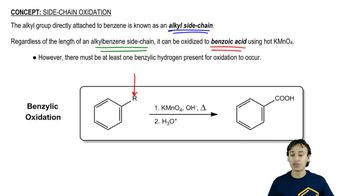
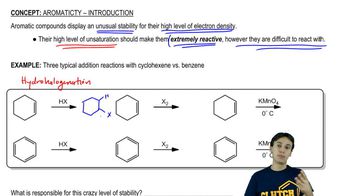
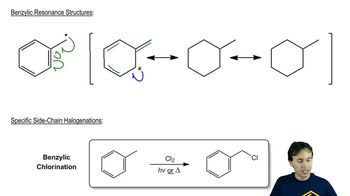
Textbook Question
The bombardier beetle defends itself by spraying a hot quinone solution from its abdomen. This solution is formed by the enzyme-catalyzed oxidation of hydroquinone by hydrogen peroxide. Write a balanced equation for this oxidation.
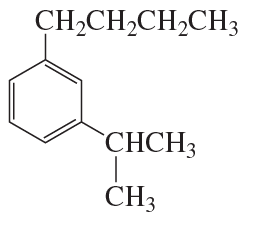
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: