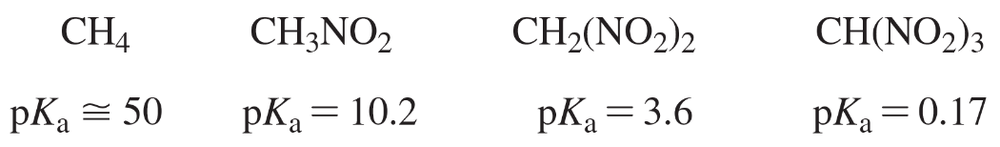Protonated cyclohexylamine has a Ka = 1 * 10-11. Using the same sequence of steps as in Problem 94, determine which is a stronger base: cyclohexylamine
or aniline.
e. Which has a greater Ka: cyclohexylammmonium ion or anilinium ion?
f. Which is a stronger acid: cyclohexylamine or aniline?