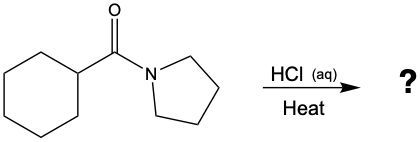
N-Acetylglucosamine (also known as NAG) is an important component on the surfaces of cells.
b. Draw the structures of the products of acid hydrolysis.
 Verified step by step guidance
Verified step by step guidance
 1:12m
1:12mMaster Acidic Hydrolysis Concept 1 with a bite sized video explanation from Jules
Start learning
