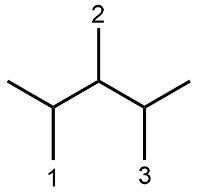
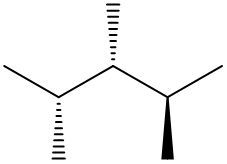
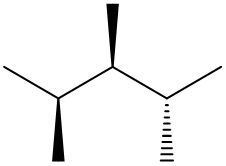
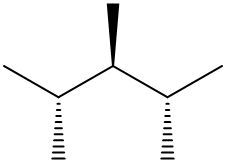
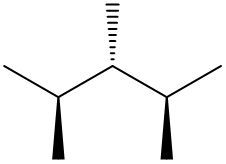
Understanding spatial orientation in molecular structures is crucial for visualizing how atoms are arranged in three-dimensional space. In skeletal formulas, the representation of bonds provides insight into both the connectivity of atoms and their spatial arrangement. Specifically, a solid wedge indicates that an atom or group is protruding out of the plane of the paper, towards the observer. For instance, if an oxygen atom (O) is depicted with a solid wedge, it suggests that the atom is oriented directly towards the viewer.
Conversely, a dashed wedge signifies that an atom or group is positioned behind the plane of the paper, away from the observer. For example, if a methyl group (CH3) is represented with a dashed wedge, it indicates that this group is oriented below the plane, moving away from the viewer.
To visualize this concept, imagine a piece of paper where the solid wedge points towards your face, while the dashed wedge extends away from you. This three-dimensional perspective is essential for accurately interpreting molecular geometry and understanding how different groups interact in a chemical context. As you delve deeper into skeletal formulas, recognizing these types of bonds will enhance your comprehension of molecular structures and their implications in chemistry.