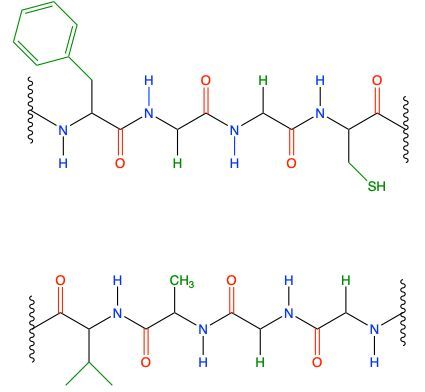
The secondary protein structure is primarily defined by the hydrogen bonding that occurs between the backbone atoms of a protein. This structure arises from interactions between the amide hydrogen of one peptide bond and the carbonyl oxygen of another. In this context, the peptide chains can be visualized as having distinct components: the amide group, which consists of the nitrogen (NH) connected to a carbonyl (C=O), and the carbonyl oxygen itself. These components facilitate the formation of hydrogen bonds, which are depicted as dashed lines in structural diagrams, indicating that they are intermolecular forces rather than true covalent bonds.
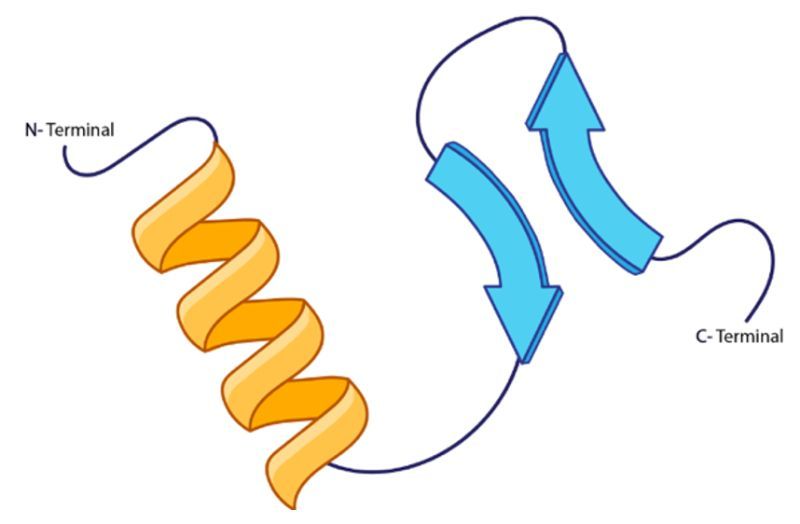
For instance, in a given peptide chain, the amide hydrogen can form a hydrogen bond with the carbonyl oxygen of an adjacent peptide chain. This interaction is crucial for stabilizing the secondary structure, which can manifest in forms such as alpha helices or beta sheets. Each hydrogen bond contributes to the overall stability and conformation of the protein, allowing it to achieve its functional shape. It is important to note that while the specific R groups of the amino acids may influence the overall structure, they do not directly participate in the hydrogen bonding that defines the secondary structure.
In summary, the secondary protein structure is a result of hydrogen bonding between the backbone components of peptide chains, specifically the amide and carbonyl groups, which play a vital role in maintaining the protein's integrity and functionality.