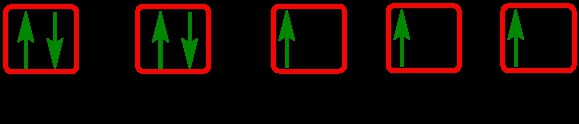
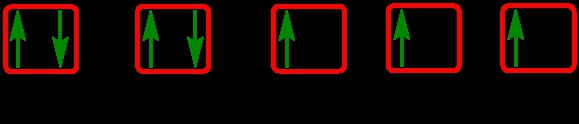
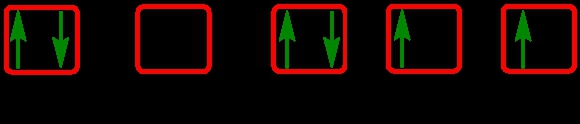
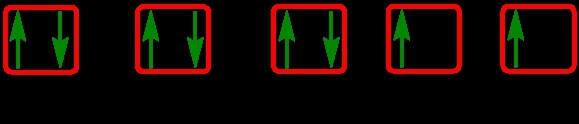
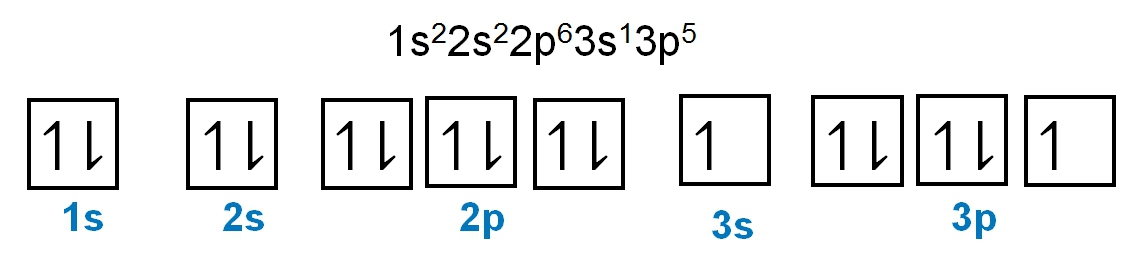
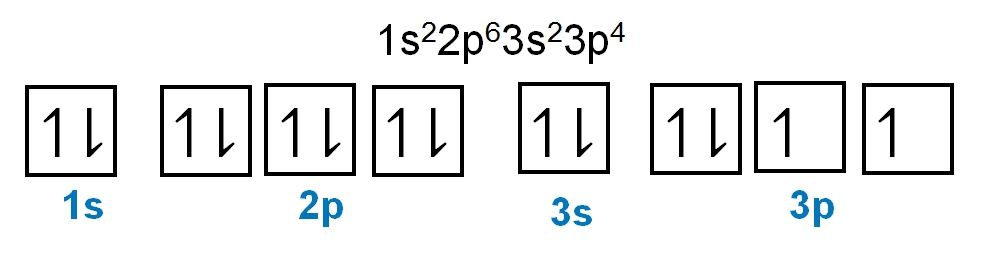
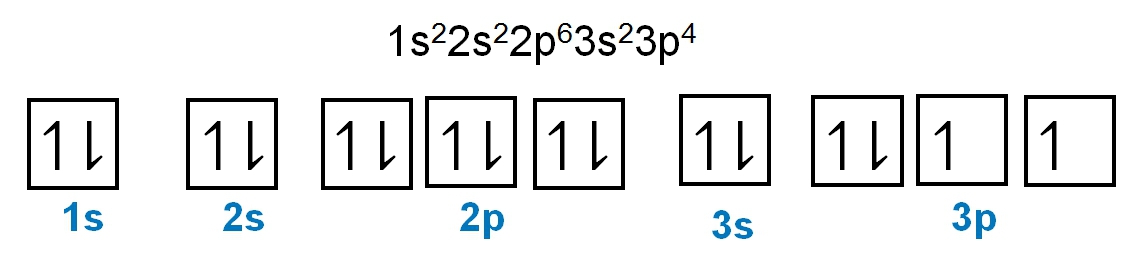
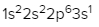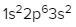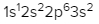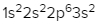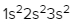The periodic law plays a crucial role in determining the electron arrangements of elements, which can be visually represented through electron orbital diagrams. These diagrams illustrate the distribution of electrons within various orbitals, highlighting the concept of degenerate orbitals—those that share the same energy level. According to Hund's rule, these degenerate orbitals are filled in a specific manner: they are first half-filled before any orbital is completely filled.
To understand the filling of these orbitals, we can examine the different sublevels:
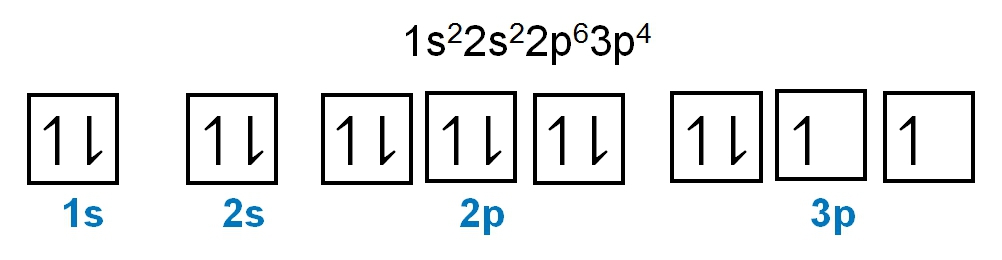
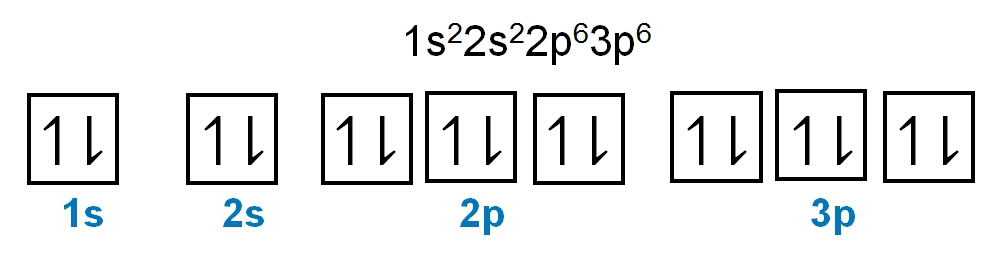
1. **s Sublevel**: The s sublevel consists of 1 orbital and can accommodate a maximum of 2 electrons. In this orbital, one electron spins up while the other spins down, adhering to the Pauli exclusion principle.
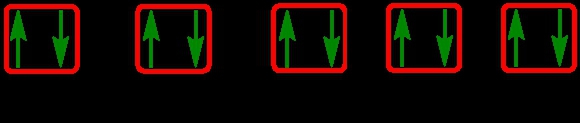
2. **p Sublevel**: The p sublevel contains 3 orbitals, allowing for a maximum of 6 electrons. Following Hund's rule, each of the three orbitals is first half-filled with one electron (all spinning up) before pairing occurs, resulting in the configuration of up, up, up, down, down, down.
3. **d Sublevel**: The d sublevel has 5 orbitals, which can hold a total of 10 electrons. Similar to the p sublevel, these orbitals are half-filled first, with one electron in each orbital before pairing begins, leading to a filling sequence of up, up, up, up, up, down, down, down, down, down.
4. **f Sublevel**: The f sublevel comprises 7 orbitals, with a maximum capacity of 14 electrons. Again, following Hund's rule, these orbitals are half-filled first before any pairing occurs, resulting in a filling order of up, up, up, up, up, up, up, down, down, down, down, down, down, down.
In summary, the periodic law significantly influences the electron arrangement of elements, and understanding the filling order of the s, p, d, and f sublevels is essential. Remember that the maximum number of electrons for each sublevel is as follows: s can hold 2, p can hold 6, d can hold 10, and f can hold 14.