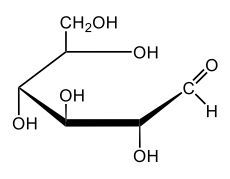
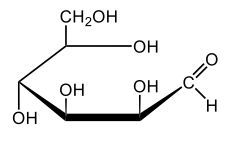
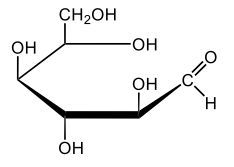
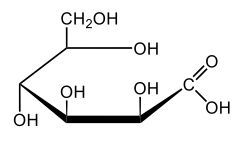
In aqueous solutions, monosaccharides exist in a dynamic equilibrium between their hemiacetal forms and the open chain form, a process known as mutarotation. This phenomenon involves the interconversion between the alpha and beta anomers of sugars through repeated ring opening and closing. The acyclic form, or open chain form, can close to form either the alpha or beta ring structures. Additionally, one of these ring forms can open to revert to the non-cyclic form and then close again to create the alternate ring form.
Central to this process is the anomeric carbon, which is carbon number 1 in the sugar structure. In the open form of D-glucose, this carbon is part of an aldehyde group, featuring a double bond to oxygen and a hydrogen atom. This open-chain form is the least stable configuration in aqueous environments, existing only in trace amounts. In contrast, the cyclic forms exhibit greater stability, with approximately 36% of D-glucose present as the alpha form and about 64% as the beta form. This distribution indicates that the beta anomer of D-glucose is more stable than the alpha form, making beta D-glucose the predominant structure in aqueous solutions, followed by the alpha form, and finally the open chain form, which is the least stable of the three.