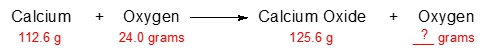The law of conservation of mass, established by the French chemist Antoine Lavoisier in the late 18th century, is a fundamental principle in chemistry. This law asserts that in a chemical reaction, matter is neither created nor destroyed; instead, it undergoes transformation from one form to another. In a typical chemical reaction, the substances present before the reaction occurs are referred to as reactants, while the substances formed as a result of the reaction are known as products. For example, in the reaction between hydrogen (H2) and oxygen (O2) to produce water (H2O), H2 and O2 are the reactants, and H2O is the product.
According to Lavoisier's law, the total mass of the reactants must equal the total mass of the products. If we consider a scenario where the combined mass of the reactants is 100 grams, this means that after the reaction, the mass of the products will also be 100 grams. Thus, all the mass from the reactants is conserved and transformed into the products. This principle is crucial for understanding various concepts in chemistry, including stoichiometry, which involves calculating the quantities of reactants and products in chemical reactions, and solution chemistry, which deals with the properties of solutions.
In summary, the law of conservation of mass is essential for predicting the outcomes of chemical reactions, as it ensures that the mass remains constant throughout the process. This foundational concept not only reinforces the idea of mass balance in reactions but also serves as a stepping stone for more complex chemical calculations and theories.