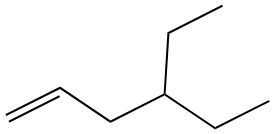
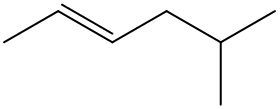
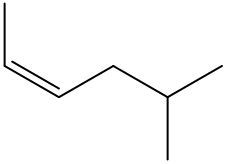
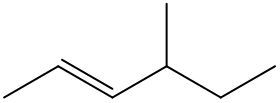
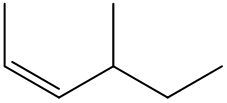
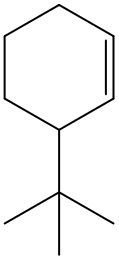
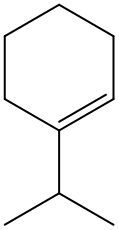
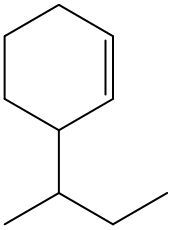
Alkenes are hydrocarbons characterized by the presence of a carbon-carbon double bond (C=C). When naming alkenes, the suffix of the parent alkane is modified from -ane to -ene, reflecting the double bond's presence. This naming convention also requires specifying the location of the double bond and any substituents attached to the carbon chain.
Geometric isomerism is a key concept in alkenes, arising from the restricted rotation around the double bond due to the formation of a pi bond. This restriction leads to different spatial arrangements of substituents, which can be classified as either cis or trans. The terms cis and trans describe the relative positions of substituents around the double bond. For a compound to exhibit cis or trans isomerism, it must have two different groups attached to the double-bonded carbons.
In a cis configuration, both substituents are located on the same side of the double bond, while in a trans configuration, they are positioned on opposite sides. For example, if two methyl groups are attached to the double-bonded carbons, and both are on the same side, the compound is classified as cis. Conversely, if one methyl group is above and the other is below the double bond, the compound is classified as trans.
It is important to note that when dealing with cyclic alkenes, the cis/trans nomenclature does not apply, as the ring structure does not allow for the same spatial orientation considerations. In summary, understanding the naming conventions and geometric isomerism of alkenes is crucial for accurately describing their structure and properties.