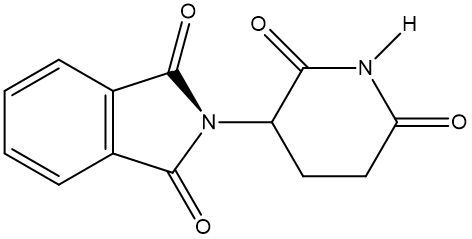
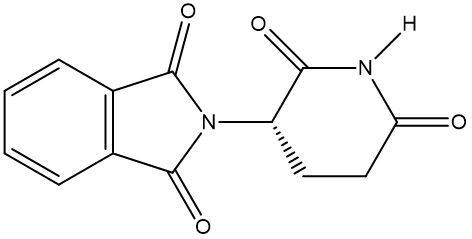
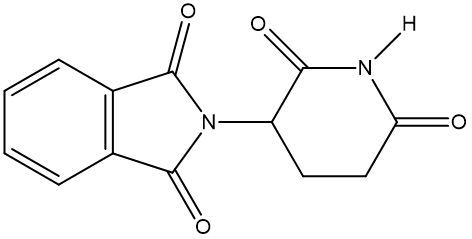
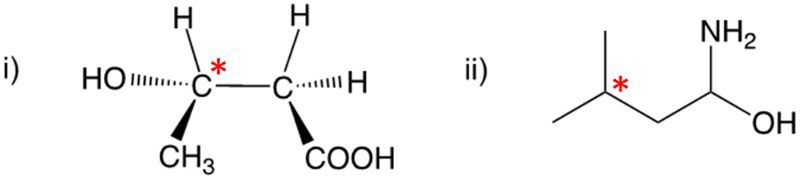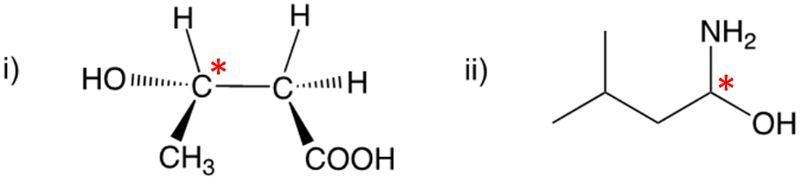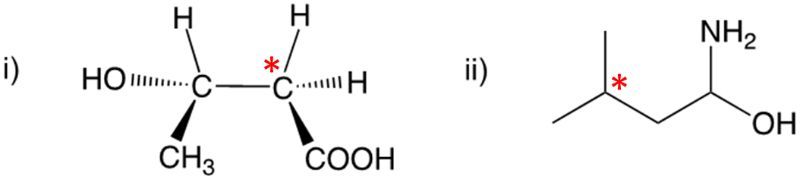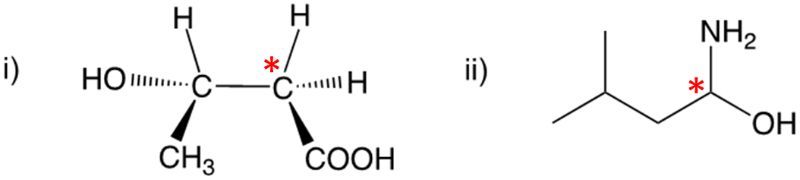Chirality is a fundamental property of certain molecules where their mirror images cannot be superimposed onto themselves. This concept can be illustrated with an analogy of a dog looking into a mirror; the reflection represents a chiral molecule, and if you attempt to align the two, they will not match due to differences in their spatial arrangement. This non-superimposable nature is crucial in understanding optical isomers, also known as enantiomers, which are a type of chiral molecule.
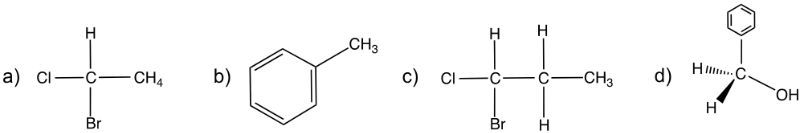
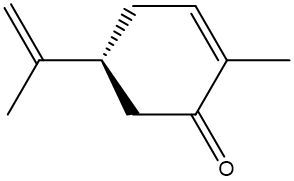
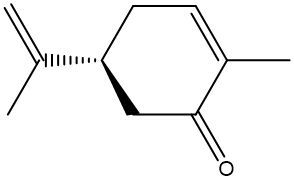
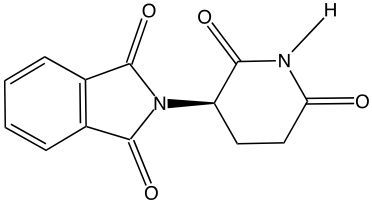
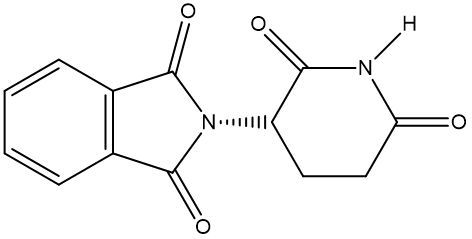
Enantiomers possess one or more chiral centers, which are specific carbon atoms bonded to four distinct groups. A carbon atom that is connected to fewer than four unique groups is classified as achiral. For example, a molecule with a carbon connected to an -OH, -NH2, and two -CH3 groups is achiral because it lacks the necessary four unique attachments. In contrast, a chiral molecule may have a carbon connected to an -OH, -NH2, a hydrogen atom, and a -CH3 group, fulfilling the requirement for chirality.
Chiral molecules are described as optically active, meaning they can rotate plane-polarized light. This property is significant in advanced organic chemistry, particularly in courses like Organic Chemistry I and II, where the behavior of these molecules in light is explored in greater detail. For now, it is essential to understand that chirality relates to the unique arrangement of atoms around a chiral center, leading to the formation of optical isomers that exhibit distinct properties due to their non-superimposable nature.