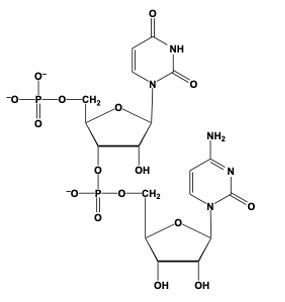
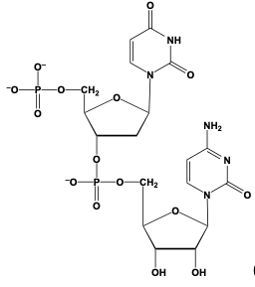
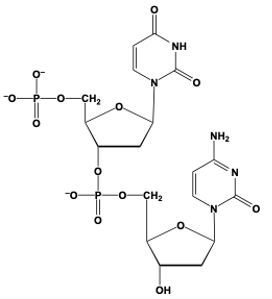
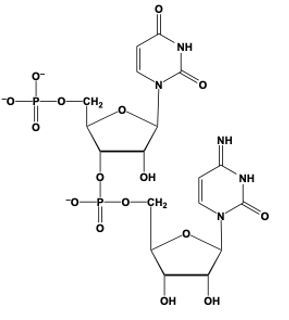
Phosphodiester bond formation is a crucial process in the structure of nucleic acids, such as DNA and RNA. This bond occurs when the hydroxyl (OH) group on the 3' carbon of one nucleotide reacts with the phosphate group of another nucleotide, resulting in the formation of an ester bond. Specifically, the 3' carbon of the sugar in one nucleotide replaces an oxygen atom in the phosphate group of another nucleotide, creating a stable linkage.
To visualize this, consider a nucleotide with a pentose sugar, which consists of a five-carbon ring. The nitrogenous base can be represented simply as "N" within a box for clarity. The 5' carbon of this nucleotide is still attached to its phosphate group. During the formation of the phosphodiester bond, the OH group on the 3' carbon of the first nucleotide interacts with one of the oxygen atoms in the phosphate group of the second nucleotide. This reaction effectively links the two nucleotides together, allowing for the continuation of the nucleic acid chain.
In summary, the formation of phosphodiester bonds is essential for connecting nucleotides, enabling the construction of long strands of DNA and RNA. This process highlights the importance of the 3' carbon's hydroxyl group in facilitating the reaction with the phosphate group, ultimately leading to the polymerization of nucleotides into nucleic acids.