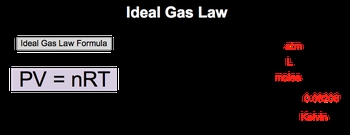
The ideal gas law, represented by the equation PV = nRT, is a fundamental equation in chemistry that relates pressure (P), volume (V), number of moles (n), the ideal gas constant (R), and temperature (T). To effectively use this equation, it is crucial to ensure that all variables are expressed in the correct units.
First, pressure must be converted to atmospheres. In this case, we start with a pressure of 600 millimeters of mercury (mmHg). The conversion factor is that 1 atmosphere is equivalent to 760 mmHg. Therefore, the conversion is performed as follows:
P = \frac{600 \text{ mmHg}}{760 \text{ mmHg/atm}} \approx 0.789 \text{ atm}
Next, the volume must be in liters. Given a volume of 500 milliliters, we convert this to liters by recognizing that 1 milliliter equals 0.001 liters:
V = 500 \text{ mL} \times 0.001 \text{ L/mL} = 0.500 \text{ L}
For the number of moles, we need to convert the mass of nitrogen gas (N2) into moles. The molar mass of nitrogen gas is calculated as follows: each nitrogen atom has a mass of 14.01 grams, and since nitrogen gas is diatomic (N2), the molar mass is:
M = 2 \times 14.01 \text{ g} = 28.02 \text{ g/mol}
Thus, to find the number of moles from 29.3 grams of N2, we use the formula:
n = \frac{29.3 \text{ g}}{28.02 \text{ g/mol}} \approx 1.05 \text{ moles}
Finally, temperature must be expressed in Kelvin. To convert from Celsius to Kelvin, we add 273.15 to the Celsius temperature. For a temperature of 50 degrees Celsius, the conversion is:
T = 50 + 273.15 = 323.15 \text{ K}
In summary, for the ideal gas law, the necessary units are:
- Pressure (P) in atmospheres: 0.789 atm
- Volume (V) in liters: 0.500 L
- Number of moles (n): 1.05 moles
- Temperature (T) in Kelvin: 323.15 K
Understanding these conversions and the correct units is essential for accurately applying the ideal gas law in various chemical calculations.