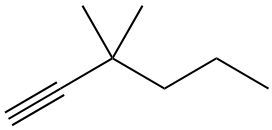
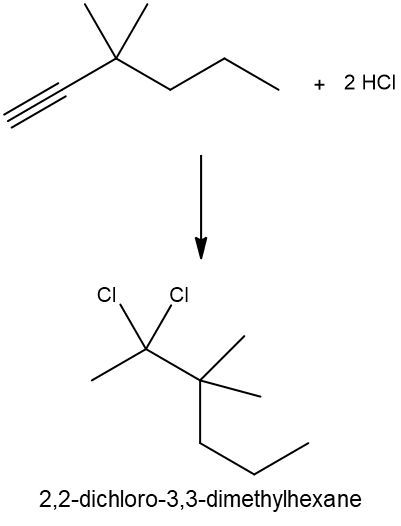
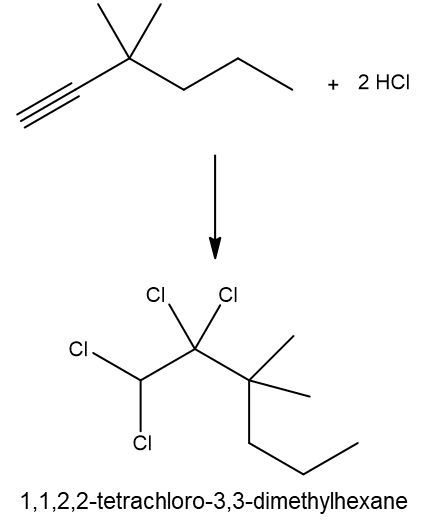
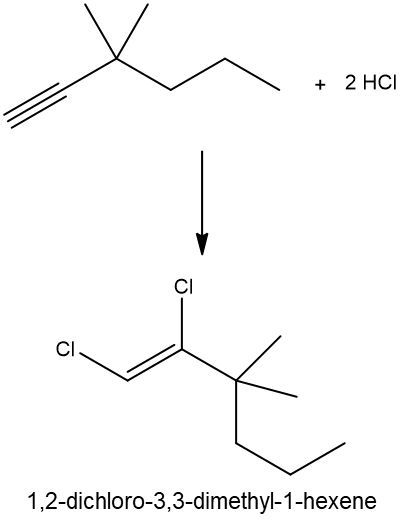
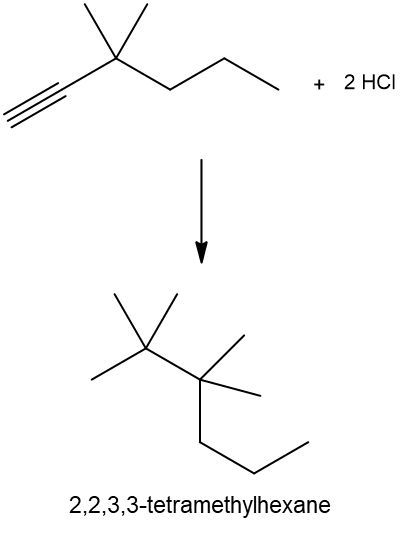
Hydrohalogenation reactions involve the addition of hydrogen (H) and a halogen (X), such as bromine or chlorine, to a pi bond in an alkene. In this process, the alkene reacts with a hydrogen halide (HX), resulting in the formation of an alkyl halide. The general reaction can be represented as:
Alkene + HX → Alkyl Halide
During the reaction, one hydrogen atom is added to one of the carbon atoms involved in the double bond, while the halogen is added to the other carbon. In symmetrical alkenes, where both carbon atoms have the same number of hydrogen atoms, the addition can occur at either carbon. For example, if we have an alkene with two equivalent carbons, the hydrogen can be added to either carbon, and the halogen will occupy the remaining position, leading to the formation of an alkyl halide product.
This transformation is significant in organic chemistry as it allows for the conversion of alkenes into more functionalized compounds, specifically alkyl halides, which can serve as intermediates in further chemical reactions.