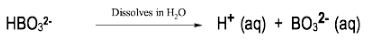
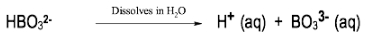
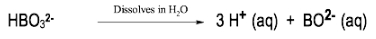
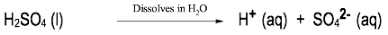In the study of acids and bases, it is essential to understand the different classifications and definitions that have been developed over time. One of the earliest and most general definitions comes from Svante Arrhenius, who proposed a framework in the late 19th century. According to Arrhenius, an acid is defined as a compound that increases the concentration of hydrogen ions (H+) when dissolved in water, while a base increases the concentration of hydroxide ions (OH-) in the same solvent.
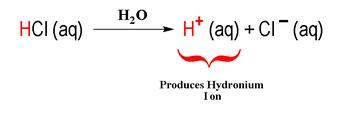
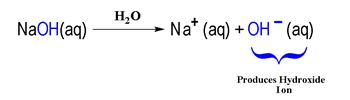
For example, hydrochloric acid (HCl) is an Arrhenius acid because it dissociates in water to produce H+ and chloride ions (Cl-). Similarly, sodium hydroxide (NaOH) is an Arrhenius base as it dissociates in water to yield sodium ions (Na+) and hydroxide ions (OH-). This definition, while foundational, has limitations; it only applies to substances in aqueous solutions and does not account for acid-base behavior in non-aqueous environments.
Arrhenius's approach categorizes acids and bases based solely on the presence of H+ and OH- ions, which can lead to an incomplete understanding of acid-base chemistry. For instance, compounds that do not contain H or OH may still exhibit acidic or basic properties, indicating the need for more comprehensive definitions that will be explored later.
As you engage with this material, consider how different compounds dissociate in water and whether they fit the Arrhenius definitions of acids or bases. This foundational knowledge will prepare you for more advanced concepts in acid-base chemistry.