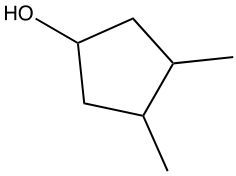
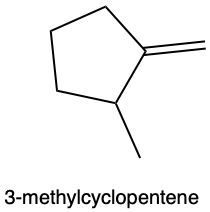
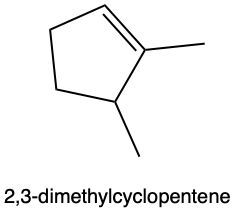
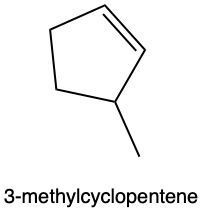
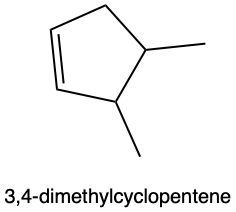
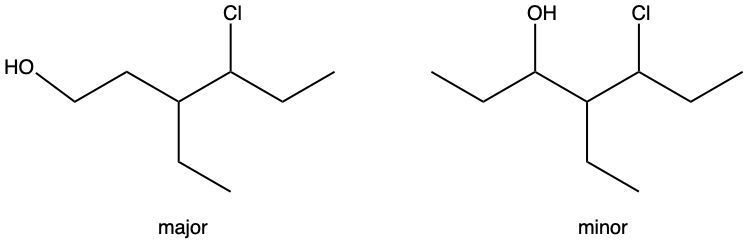
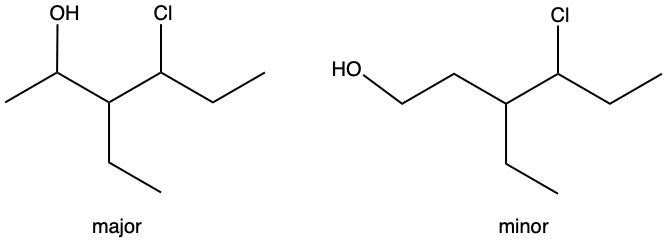
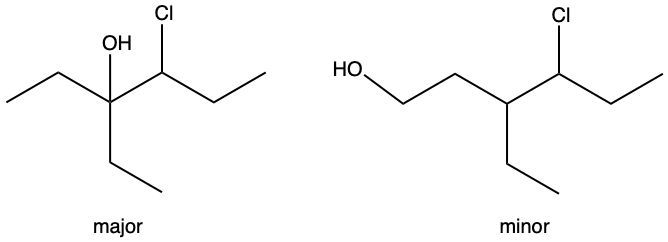
Hydration reactions involve the acid-catalyzed addition of water to an alkene, resulting in the formation of an alcohol. In this process, one hydrogen atom (H) and one hydroxyl group (OH) from water are added across the pi bond of the alkene. The reaction requires sulfuric acid, which serves as an acid catalyst to initiate the reaction.
When examining a symmetrical alkene, both double-bonded carbons are equivalent, each connected to one carbon and one hydrogen atom. This symmetry allows for the H and OH to be added to either of the double-bonded carbons. In the reaction, the H is added to one carbon, while the OH is added to the other, effectively breaking the double bond and resulting in a saturated alcohol product.
It is important to note that the outcome of the hydration reaction can differ when dealing with unsymmetrical alkenes. In such cases, the placement of the H and OH groups will depend on the specific structure of the alkene, which can lead to different alcohol products. Overall, the key takeaway is that hydration reactions are a fundamental method for converting alkenes into alcohols through the addition of water, facilitated by an acid catalyst.