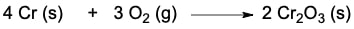Balancing chemical equations is a crucial first step in understanding stoichiometry, which focuses on the quantitative relationships between reactants and products in a chemical reaction. Stoichiometry enables us to calculate the amounts of products formed from given reactants and vice versa. For instance, consider the balanced chemical equation:
2 H2 (g) + O2 (g) → 2 H2O (g)
In this example, 12.3 grams of hydrogen gas (H2) are provided, and the goal is to determine the mass of water (H2O) produced. This process exemplifies stoichiometry, where we use the balanced equation to relate the quantities of different substances involved in the reaction.
To perform stoichiometric calculations, one must follow specific procedures that involve converting grams to moles, using the mole ratio from the balanced equation, and then converting back to grams if necessary. Understanding these steps is essential for accurately determining the amounts of reactants and products in any chemical reaction.