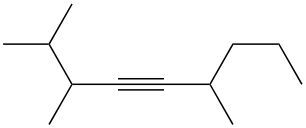
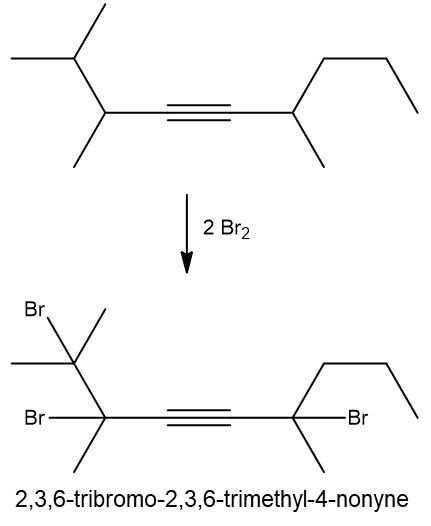
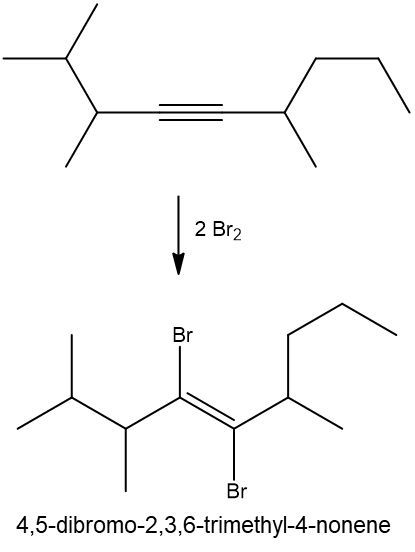
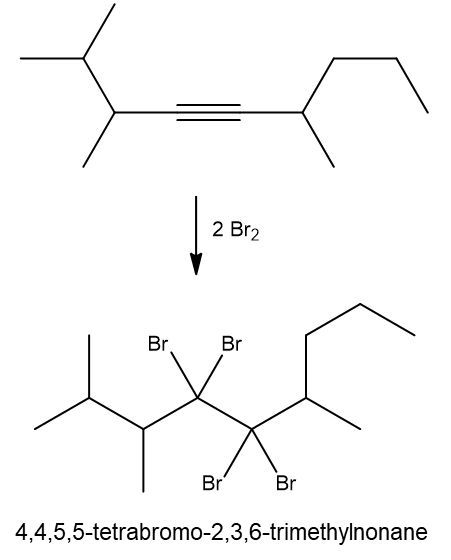
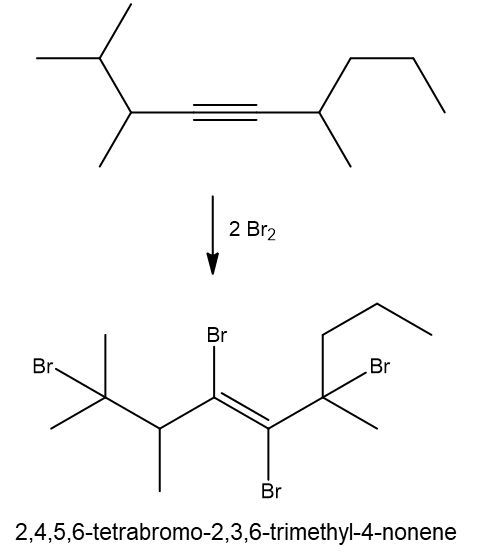
Halogenation reactions involve the addition of halogens, such as bromine (Br2) or chlorine (Cl2), to alkenes and alkynes. In these reactions, each double bond (π bond) in the starting alkene reacts with one mole of halogen, resulting in the formation of a dihalide. This means that for every π bond present, one mole of halogen is required, leading to the addition of two halogen atoms to the molecule.
For example, when an alkene undergoes halogenation, the two carbon atoms involved in the double bond each gain one halogen atom, resulting in a compound with two halogens, known as a dihalide. Conversely, in the case of an alkyne, which contains two π bonds, two moles of halogen are necessary. The first mole adds two halogens, and the second mole adds another two, yielding a compound with four halogen atoms, referred to as a tetrahalide.
In summary, halogenation is characterized by the addition of two halogens per π bond in the structure, producing either a dihalide from alkenes or a tetrahalide from alkynes.