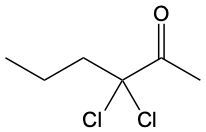
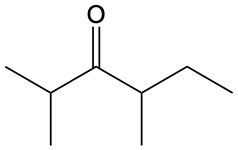
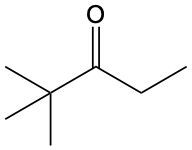
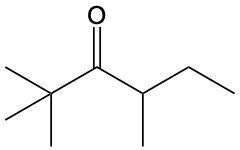
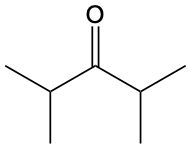
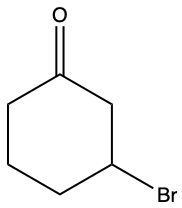
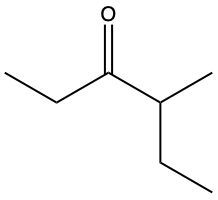
Ketones are organic compounds characterized by a carbonyl group (C=O) where the carbonyl carbon is bonded to two other carbon atoms. The naming of ketones follows a systematic approach similar to that of alcohols, with a key distinction in the suffix used. Specifically, the 'e' at the end of the alkane name is replaced with 'one' to indicate the presence of a ketone. For example, the ketone derived from pentane is named pentanone.
When naming ketones, it is essential to identify the longest carbon chain that includes the carbonyl group, as this will serve as the parent chain. The position of the carbonyl group must be indicated by a number, which corresponds to the carbon atom in the chain where the carbonyl is located. Additionally, if there are any substituents attached to the carbon chain, their positions must also be specified. This systematic approach ensures clarity in the naming of ketones, allowing for accurate communication of their structure.