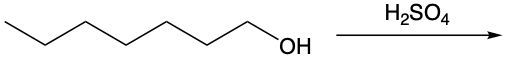
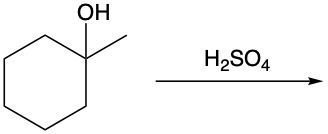
Dehydration reactions involving alcohols are a key aspect of organic chemistry, particularly in the formation of alkenes. In these reactions, sulfuric acid (H2SO4) acts as a catalyst, facilitating the conversion of an alcohol into an alkene through the elimination of water (H2O).
During the dehydration process, the hydroxyl group (–OH) from the alcohol carbon is removed, along with a hydrogen atom (H) from a neighboring carbon atom. This results in the formation of a double bond between the two carbon atoms, leading to the creation of an alkene. For instance, consider an alcohol with two adjacent methyl groups. When sulfuric acid is introduced, the –OH group is lost from one carbon, and an H atom is lost from the neighboring carbon. The removal of these components results in the release of water and the establishment of a double bond between the two carbons, thus forming the alkene product.
It is essential to remember that carbon atoms must maintain four bonds. Therefore, after losing the –OH and H, the carbon atoms will form a double bond to satisfy this tetravalency. This reaction highlights the importance of dehydration in organic synthesis, showcasing how simple alcohols can be transformed into more complex structures like alkenes through the strategic removal of water.