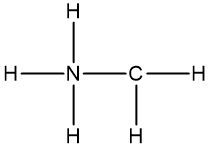
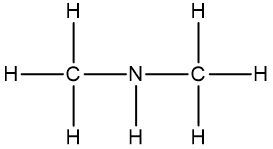
Molecular formulas, such as C2H6O, provide essential information about the number and types of atoms in a compound but fall short in conveying structural details. Understanding the structure of organic compounds is crucial as it reveals the connectivity of atoms and their spatial orientation, which is particularly important when discussing stereoisomers.
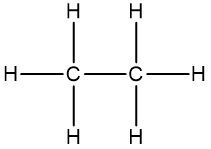
Connectivity refers to how atoms are linked within a molecule. For instance, the molecular formula C2H6O can represent different structural arrangements. One possible structure features two carbon atoms connected to each other, with five hydrogen atoms distributed among them, and an oxygen atom bonded to one of the carbons along with an additional hydrogen atom. Alternatively, the same molecular formula could depict two carbon atoms each bonded to an oxygen atom, with three hydrogen atoms attached to each carbon. Both structures are valid representations of the same molecular formula, highlighting the limitation of molecular formulas in providing a definitive structure.
Thus, while molecular formulas indicate the composition of a compound, they do not specify how the atoms are arranged or their orientation in three-dimensional space. This lack of structural information can lead to ambiguity, underscoring the need for more detailed representations, such as structural formulas, to fully understand organic compounds.